Immunogenicity assessment of swim bladder-derived biomaterial
LabEX支持文献- Biomater Sci
- 2023
- 5.7
- 2023 Apr 11;11(8)
- Mouse
- Luminex
- 免疫/内分泌
- 血清
- 免疫/内分泌
- IL-1 β/IL-1F2,IL-2,IL-4,IL-5,IL-6,IL-10,IL-12p70,CXCL1/GRO/α/KC/CINC-1,IFN-γ,TNF-α
- 10.1039/d2bm01419j
相关货号
Abstract
Fish swim bladder-derived biomaterials are prospective cardiovascular materials due to anti-calcification, adequate mechanical properties, and good biocompatibility. However, their immunogenic safety profile, which primarily determines their feasibility as medical devices in clinical practice, remains unknown. Herein, the immunogenicity of glutaraldehyde crosslinked fish swim bladder (Bladder-GA) and un-crosslinked swim bladder (Bladder-UN) samples was examined using in vitro and in vivo assays according to ISO 10993-20. The in vitro splenocyte proliferation assay showed that cell growth was lower in the extract medium of Bladder-UN and Bladder-GA, compared to the LPS-or Con A-treated group. Similar results were obtained in in vivo assays. In the subcutaneous implantation model, the thymus coefficient, spleen coefficient and ratio of immune cell subtypes showed no significant difference between the bladder groups and the sham group. In terms of the humoral immune response, the total IgM concentration was lower in the Bladder-GA and Bladder-UN groups (988 ± 238 μg ml-1 and 1095 ± 296 μg ml-1, respectively) than that in the sham group (1329 ± 132 μg ml-1) at 7 days. The total IgG concentrations were 422 ± 78 μg ml-1 in Bladder-GA and 469 ± 172 μg ml-1 in Bladder-UN at 30 days, which were slightly higher than that in the sham group (276 ± 95 μg ml-1) but there was no significant difference compared with Bovine-GA (468 ± 172 μg ml-1), indicating that these materials did not elicit a strong humoral immune response. Systemic immune response-related cytokines and C-reactive protein were stable during implantation, while IL-4 levels increased with time. The classical foreign body response was not observed around all the implants, and the ratio of CD163+/iNOS macrophages in Bladder-GA and Bladder-UN was higher than that in the Bovine-GA group at the implanted site at 7 and 30 days. Finally, no organ toxicity was observed in any of the groups. Collectively, the swim bladder-derived material did not elicit significant aberrant immune responses in vivo, giving strong confidence for its application in tissue engineering or medical devices. Furthermore, more dedicated research on immunogenic safety assessment in large animal models is encouraged to facilitate the clinical practice of swim bladder-derived materials.
LabEx ELISA平台助力心血管材料研究
本周为大家带来的文献为发表于Biomater Sci (IF: 6.6)的” Immunogenicity assessment of swim bladder-derived biomaterials”。本文使用了LabEx提供的ELISA检测服务。
随着世界范围内长寿化和老龄化社会的发展,近年来瓣膜性心脏病(VHD)的发病率不断上升,引起了人们的广泛关注。鱼鳔生物材料具有抗钙化、足够的机械性能和良好的生物相容性,是一种前景广阔的心血管材料。然而,这些材料的免疫原性安全性仍是未知数,而免疫原性安全性主要决定了其作为医疗器械在临床实践中的可行性。在此,我们根据ISO 10993-20 标准,采用体外和体内检测法对戊二醛交联鱼鳔(Bladder-GA)和未交联鱼鳔(Bladder-UN)样本的免疫原性进行了研究。
LabEx提供的ELISA检测服务:
研究者使用相应的ELISA试剂盒测定小鼠血清总IgG、IgM 和 C 反应蛋白,并使用LabEx公司的Luminex技术测定血清中细胞因子的浓度。研究者检测了皮下注射后 7 天和 30 天的血清 IgM 和 IgG 水平。7天时,膀胱-GA组(988 ± 238 μg ml-1)和膀胱-UN组(1095 ± 296 μg ml-1)小鼠的血清总IgM浓度略低于假体组(1329 ± 132 μg ml-1),两组间差异不大。可以看出,30 天时,膀胱-GA 组(422 ± 78 μg ml-1)和膀胱-UN 组(469 ± 172 μg ml-1)小鼠的血清总 IgG 浓度略高于假组(276 ± 95 μg ml-1),但与牛-GA 组(468 ± 172 μg ml-1)相似,无显著差异(下图)。
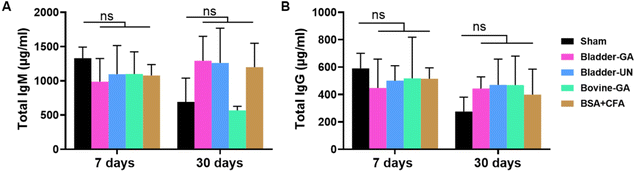
皮下注射后7天和30天,5组小鼠血清中总IgM(A)和IgG(B)的浓度
重要发现:
- 在体外脾细胞增殖实验中,Bladder-UN和Bladder-GA的细胞增殖率低于LPS或Con A处理组。
- 在体内实验中,皮下植入模型的胸腺系数、脾系数和免疫细胞亚型比例在鱼鳔组和对照组之间无显著差异。
- 体液免疫反应方面,Bladder-GA和Bladder-UN组的总IgM浓度在7天时低于对照组;在30天时,总IgG浓度略高于对照组,但与Bovine-GA组无显著差异,表明这些材料未引发强烈的体液免疫反应。
- 植入期间,全身免疫反应相关的细胞因子和C反应蛋白保持稳定,而IL-4水平随时间增加。
- 在所有植入物周围未观察到经典的异物反应,且在7天和30天时,Bladder-GA和Bladder-UN组的CD163+/iNOS巨噬细胞比例高于Bovine-GA组。
- 最后,各组均未观察到器官毒性。
本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。











 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


