Bispecific NK-cell engager targeting BCMA elicits stronger antitumor effects and produces less proinflammatory cytokines than T-cell engager
NK-cell engager; T-cell engager; antitumor; proinflammatory cytokines; tumor immunotherapy LabEX支持文献- Front Immunol
- 2023
- 5.7
- 2023 Apr 11:14:1113303
- Human
- MSD
- 肿瘤
- 血清
- 免疫/内分泌
相关货号
Abstract
Bispecific antibodies have attracted more attention in recent years for the treatment of tumors, in which most of them target CD3, which mediates the killing of tumor cells by T cells. However, T-cell engager may cause serious side effects, including neurotoxicity and cytokine release syndrome. More safe treatments are still needed to address unmet medical needs, and NK cell-based immunotherapy is a safer and more effective way to treat tumors. Our study developed two IgG-like bispecific antibodies with the same configuration: BT1 (BCMA×CD3) attracted T cells and tumor cells, while BK1 (BCMA×CD16) attracted NK cells and tumor cells. Our study showed that BK1 mediated NK cell activation and upregulated the expression of CD69, CD107a, IFN-γ and TNF. In addition, BK1 elicited a stronger antitumor effect than BT1 both in vitro and in vivo. Combinatorial treatment (BK1+BT1) showed a stronger antitumor effect than either treatment alone, as indicated by in vitro experiments and in vivo murine models. More importantly, BK1 induced fewer proinflammatory cytokines than BT1 both in vitro and in vivo. Surprisingly, BK1 reduced cytokine production in the combinatorial treatment, suggesting the indispensable role of NK cells in the control of cytokine secretion by T cells. In conclusion, our study compared NK-cell engagers and T-cell engagers targeting BCMA. The results indicated that NK-cell engagers were more effective with less proinflammatory cytokine production. Furthermore, the use of NK-cell engagers in combinatorial treatment helped to reduce cytokine secretion by T cells, suggesting a bright future for NK-cell engagers in clinical settings.
Keywords: NK-cell engager; T-cell engager; antitumor; proinflammatory cytokines; tumor immunotherapy.
LabEx MSD平台助力探索基于NK细胞的肿瘤免疫疗法
本周为大家带来的文献为发表Front Immunol. (IF: 5.7)的” Bispecific NK-cell engager targeting BCMA elicits stronger antitumor effects and produces less proinflammatory cytokines than T-cell engager”。本文使用了LabEx提供的MSD检测服务。
双特异性抗体近年来在肿瘤治疗中引起了更多关注,其中大多数靶向CD3,通过T细胞介导肿瘤细胞的杀伤。然而,T细胞接合剂可能会引起严重的副作用,包括神经毒性和细胞因子释放综合征。为了应对未满足的医疗需求,更安全的治疗方法仍然是必要的,而基于NK细胞的免疫疗法是一种更安全、更有效的肿瘤治疗方式。本文旨在探索基于NK细胞的肿瘤免疫疗法。
LabEx提供的MSD检测服务,
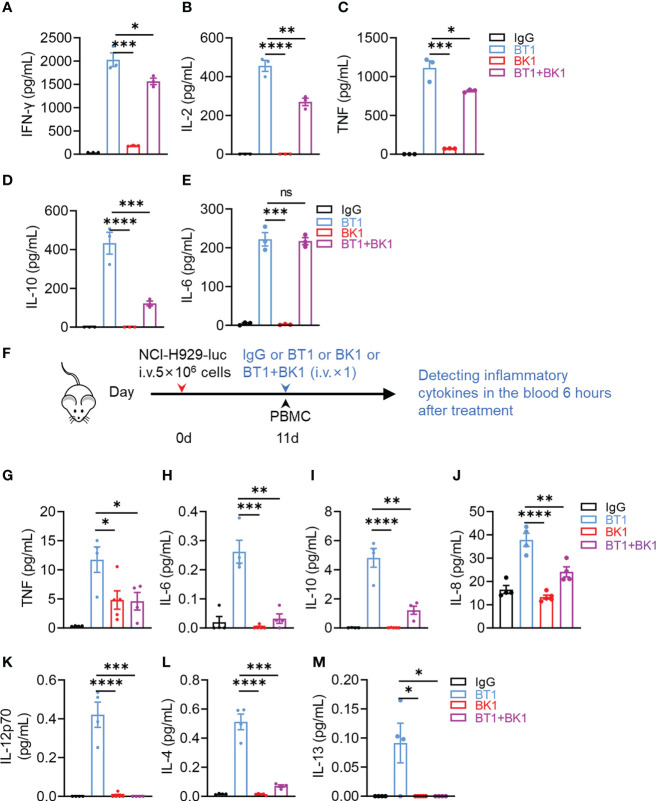
在第0天,将表达荧光素酶的NCI-H929肿瘤细胞通过静脉注射(i.v.)注入NSG小鼠。第11天,通过静脉注射(i.v.)注入外周血单个核细胞(PBMCs)(2 × 10^7)。第11天,将IgG、BT1、BK1和BT1+BK1(每只小鼠50微克)与IL-2通过静脉注射(i.v.)注入肿瘤负荷小鼠。治疗后6小时,用V-PLEX Proinflammatory Panel 1(人类)试剂盒(LXMH10-1,LabEx,上海,中国)检测血清细胞因子。
数据表明,细胞因子风暴和神经毒性是免疫疗法的阻碍因素。为了评估BT1和BK1的潜在风险,在体外实验中,将PBMCs和MM.1S-luc细胞在IgG、BK1、BT1或BK1与BT1联合存在的情况下共同培养24小时后,测定上清液中的细胞因子水平。结果表明,与BK1相比,BT1诱导的细胞因子分泌水平显著更高,包括IFN-γ、IL-2、TNF、IL-10和IL-6(图A-E,),其中IL-6、TNF和IL-10是细胞因子风暴的强诱导剂。有趣的是,与BT1相比,联合治疗的上清液中观察到显著较低水平的细胞因子,包括IL-2、IL-10和IL-6,表明BT1与BK1的联合使用减少了BT1引起的细胞因子分泌。
重要发现,
该研究开发了两种具有相同结构的IgG样双特异性抗体:BT1(BCMA×CD3)吸引T细胞和肿瘤细胞,而BK1(BCMA×CD16)吸引NK细胞和肿瘤细胞。研究显示,BK1介导NK细胞激活并上调CD69、CD107a、IFN-γ和TNF的表达。此外,BK1在体内外均表现出比BT1更强的抗肿瘤效果。联合治疗(BK1+BT1)显示出比单独治疗更强的抗肿瘤效果,这在体外实验和体内小鼠模型中均有体现。更重要的是,BK1在体内外均诱导的促炎性细胞因子比BT1少。令人惊讶的是,BK1在联合治疗中减少了细胞因子的产生,表明NK细胞在控制T细胞细胞因子分泌中起着不可或缺的作用。
该研究比较了靶向BCMA的NK细胞接合剂和T细胞接合剂。结果表明,NK细胞接合剂更有效且促炎性细胞因子产生更少。此外,联合治疗中使用NK细胞接合剂有助于减少T细胞的细胞因子分泌,这表明NK细胞接合剂在临床应用中前景光明。
本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。







 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


