Treatment with Humanized Selective CD19CAR-T Cells Shows Efficacy in Highly Treated B-ALL Patients Who Have Relapsed after Receiving Murine-Based CD19CAR-T Therapies
CART;细胞治疗;cell therapy; 急性淋巴细胞白血病- Clin Cancer Res
- 2019
- 13.801
- Mouse
- MSD
- serum
- 药物研发
- T细胞
- IL2, IFNγ, and TNFα
Abstract
Abstract
Purpose: CD19 chimeric antigen receptor (CAR)-T therapy has shown impactful results in treatment of B-cell malignancies. However, immune recognition of the murine scFv may render subsequent infusion(s) ineffective. Also, nonselective expansion of both CAR-transduced and nontransduced T cells during the production stage affects the yield and purity of final products. Here, we aim to develop a humanized selective (hs) CD19 CAR to solve the above problems.Experimental Design: A CD19 hsCAR was designed, which incorporated a short selective domain between the humanized heavy chain and light chain. The CAR was examined for its property, and then trialed in 5 highly treated B-ALL patients.Results: hsCAR possessed around 6-fold higher affinity to CD19 versus murine CAR (mCAR). Incubation with selective domain-specific mAbs (SmAb) selectively expanded CAR-transduced T cells, and led to a higher proportion of central memory T cells in the final products. SmAb-stimulated CD19 hsCAR-T cells exhibited superior antitumor cytotoxic functions in vitro and in vivo. Autologous (n = 2) and allogeneic donor (n = 3, with hematopoietic stem cell transplantation) hsCAR-T cells were infused into 5 patients who had relapsed after receiving mCAR-T treatments. Two patients received mCAR-T treatments twice previously but the second treatments were ineffective. In contrast, subsequent hsCAR-T treatments proved effective in all 5 patients and achieved complete molecular remission in four, including one with extramedullary disease with central nervous system involvement.Conclusions: hsCD19 CAR-T treatment shows efficacy in highly treated B-ALL patients who have relapsed after receiving CD19 mCAR-T therapies.LabEx 细胞因子定量技术 在肿瘤免疫治疗中的应用
PD-1/PD-L1在肺癌中的抗肿瘤中的应用
肺癌是世界范围内癌症相关死亡的主要原因,非小细胞肺癌(NSCLC)约占所有病例的80%。因此,为了让更多的人群受益,肺癌迫切需要发展免疫治疗与常规治疗相结合等创新治疗方法。其中测定循环和瘤内IL-10、IFN-γ、TNF-α、GM-CSF使用MSD技术的U-plex生物标志物组。

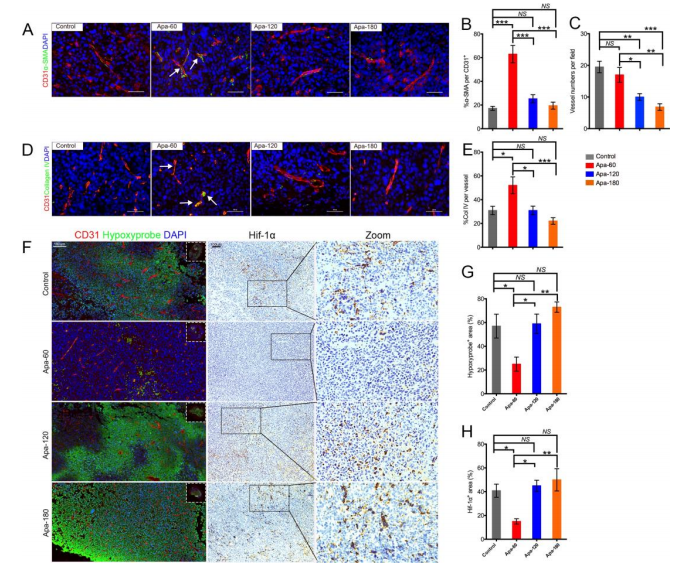
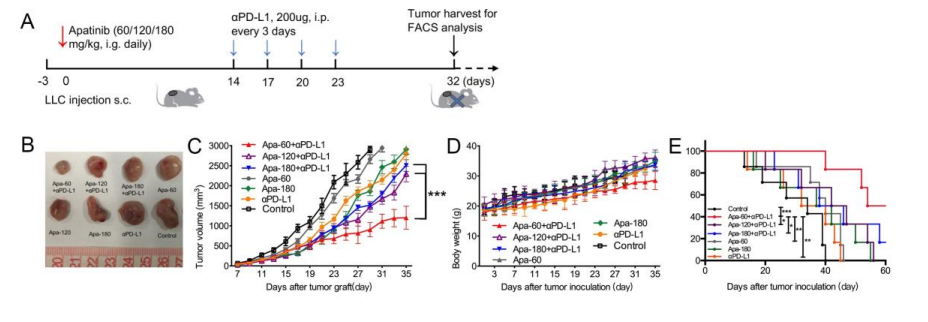
基于同基因肺癌小鼠模型,本研究证明了低剂量的阿帕替尼可缓解缺氧,增加CD8+ T细胞的浸润,减少肿瘤中肿瘤相关巨噬细胞(TAMs)的募集并减少肿瘤及血清中TGF-β水平变化。小剂量阿帕替尼联合抗PD-L1抗体可显著延缓肿瘤生长和转移,延长小鼠模型的生存时间。
MSD技术在CAR-T细胞治疗研究中的应用
CAR-T疗法就是嵌合抗原受体T细胞免疫疗法,英文全称Chimeric Antigen Receptor T-Cell Immunotherapy。这是一种治疗肿瘤的新型精准靶向疗法。这些临床试验中使用的单链抗体序列是基于小鼠抗体序列,最近的研究证实,宿主免疫反应可以识别小鼠单链抗体的表位,并使随后的治疗无效。在本研究中,开发了一种人源化的CD19 CAR,并将10个氨基酸的E-tag作为scFv重链和轻链之间的选择域,hsCD19 CAR-T治疗在CD19 mCAR-T疗法治疗后复发的B-ALL患者中显示出疗效。

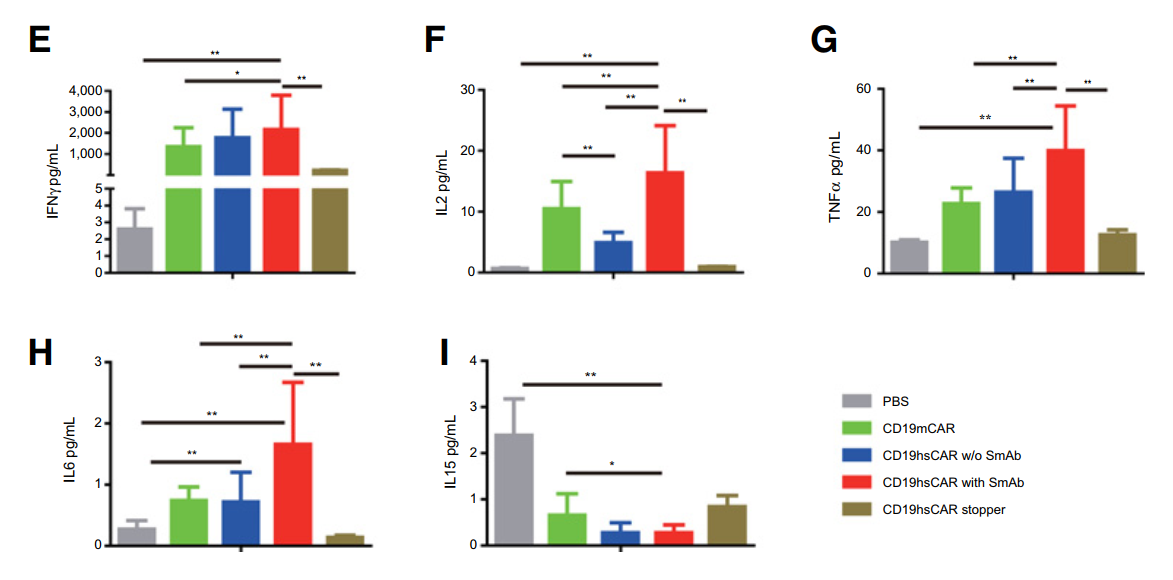
在小鼠白血病模型中,以正常健康人外周血单核细胞为研究对象,产生不同类型的外周血单核细胞CD19 CAR- t细胞,包括mCAR, hsCAR和对照hsCAR停止CAR,进行干预治疗。小鼠血清中细胞因子含量检测方式: MSD MULTI-SPOT Assay system (Meso Scale Diagnostics) 。用SmAb激活的hsCAR-T细胞治疗的小鼠显示出更长的生存期,显示出更高的促炎细胞因子(IFN-, IL-2, TNF- IL-6))浓度和更低的IL-15的浓度。
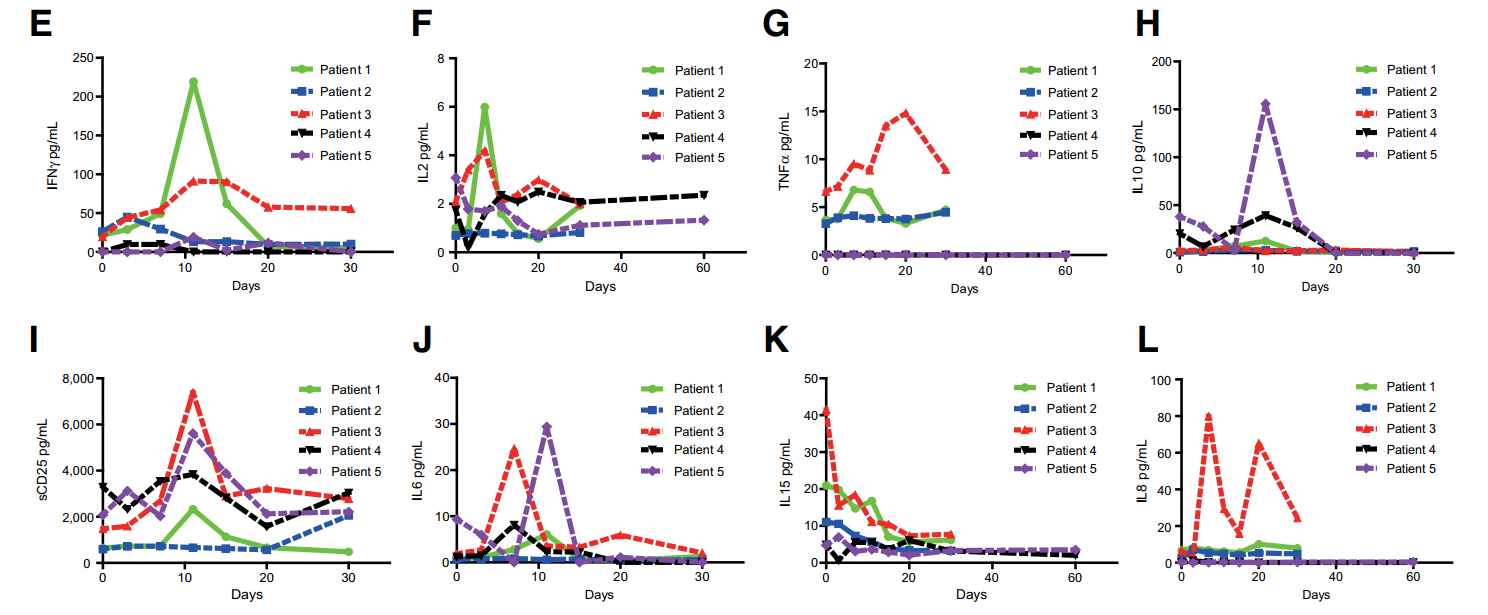
为了评估hsCAR-T细胞疗法的安全性,本研究检测5名患者血清中细胞因子的表达情况。人血清中细胞因子含量检测方式:Panel 1 (human) Kit of MSD MULTI-SPOT Assay system (Meso Scale Diagnostics)。患者1和患者3血清中检测到IL-2、IL-6、sCD25和TNF水平激增,在患者4和患者5的IL-6水平明显升高。
综上所述,研究结果证实了肠道菌群与血浆Aβ42/Aβ40可作为临床前AD的筛选工具而针对肠道菌群可能会为AD相关认知功能下降的治疗策略提供新的思路。
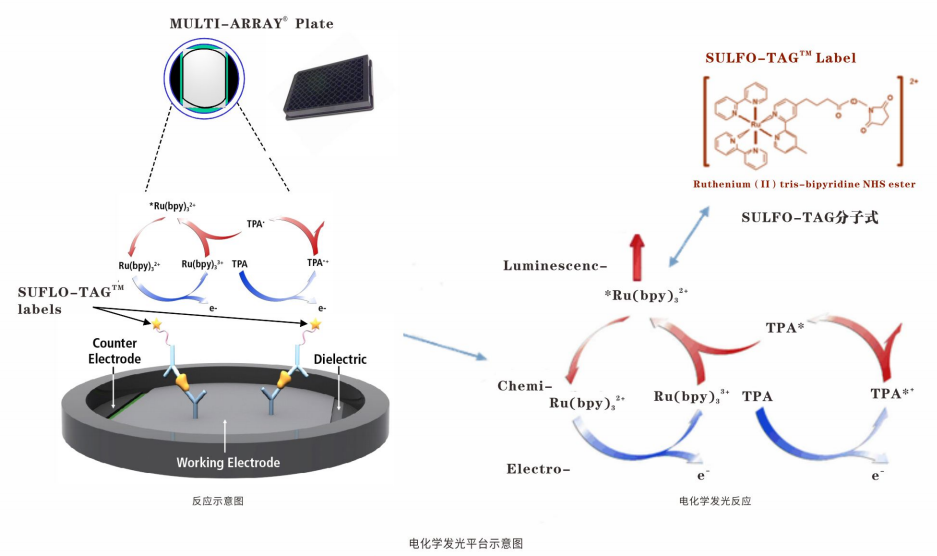
以上两篇文章中都有用到MSD电化学发光技术,该技术是基于ELISA基本原理的升级,在板底通电从而激发标记物SULFO-TAG发光并由CCDCamera进行信号采集;MSD ECL技术大大提高免疫分析的灵敏度,延伸了线性范围。MSD通 过点阵技术,在96孔石墨电极板里可实现10个指标/每孔的检测,同时实现多个指标的相对或绝对定量。
MSD电化学发光技术独具一格的“ 六合一”特点:
亚fg/ml灵敏度,低背景,多靶点检测,6个数量级线性范围,适用于不同样本基质 ( 血清/血浆, 细胞培养上清,脑脊液,尿液,组织匀浆,细胞裂解液)(人/猴子/小鼠/大鼠),5-25ul微量样本检测。
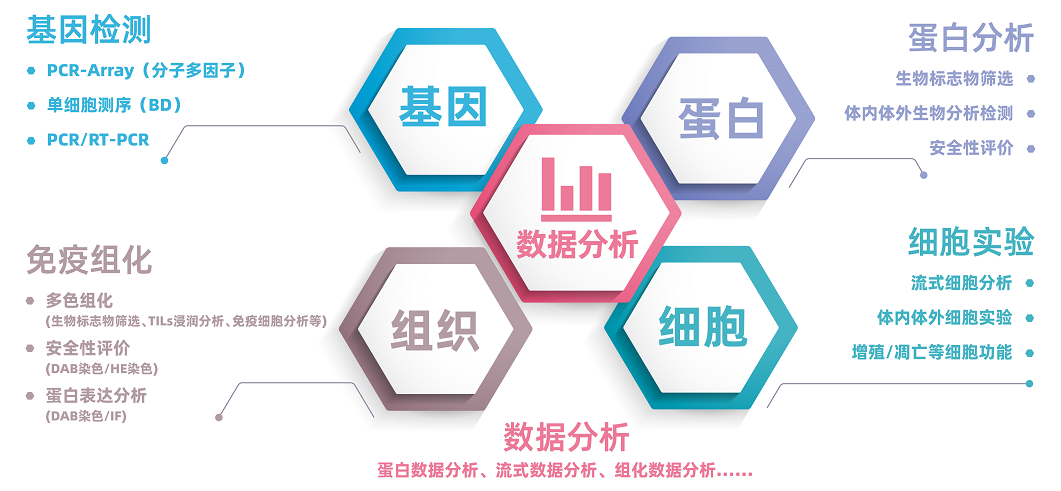
LabEx作为多因子实验服务专家,可以提供MSD/Luminex/CBA等多因子检测服务,速度快,质量高,服务更专业。LabEx每年检测25万+多因子样本,并可提供专业的生物信息学分析,以帮助用户获得更高质量的检出数据和服务体验。此外,LabEx还可以提供以下服务平台:
本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。







 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


