Integrin αvβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy
177Lu-EB-RGD;Targeted radionuclide therapy;combination treatment;immunotherapy;programed death ligand 1 (PD-L1) LabEX支持文献- Theranostics
- 2019
- 11.6
- 9(25):7948-7960.
- Mouse
- HE染色
- mouse tissues
- 免疫/内分泌
- 肿瘤
Abstract
Methods: To explore the most appropriate timing of immunotherapy after radionuclide therapy, the anti-PD-L1 antibody (αPD-L1 mAb) was delivered in a concurrent or sequential manner when 177Lu TRT was given.
Results: The results demonstrated that TRT led to an acute increase in PD-L1 expression on T cells, and TRT in combination with αPD-L1 mAb stimulated the infiltration of CD8+ T cells, which improved local tumor control, overall survival and protection against tumor rechallenge. Moreover, our data revealed that the time window for this combination therapy may be critical to outcome.
Conclusions: This therapeutic combination may be a promising approach to treating metastatic tumors in which TRT can be used. Clinical translation of the result would suggest that concurrent rather than sequential blockade of the PD-1/PD-L1 axis combined with TRT improves overall survival and long-term tumor control.
LabEx Luminex平台助力肿瘤疗法研究
本周为大家带来的文献为发表于Theranostics. (IF:12.4)上的《Integrin αvβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy》。本文使用了LabEx提供的Luminex检测服务。
本文探讨了联合使用放射性核素治疗(TRT)和免疫检查点阻断疗法(ICB)对提高抗肿瘤疗效的可能性。在这项研究中,研究人员提出了一种新疗法,即在小鼠结肠癌模型中结合使用基于程序性死亡配体1(PD-L1)的免疫疗法和基于肽的TRT(以177Lu为放射性核素)。通过探索在放射性核素治疗后给予抗PD-L1抗体(αPD-L1 mAb)的最佳时机,研究发现TRT会导致T细胞上PD-L1表达的急性增加,而TRT与αPD-L1 mAb的结合刺激了CD8+ T细胞的浸润,从而提高了局部肿瘤控制、总生存期和对肿瘤再挑战的保护。此外,数据揭示这种联合疗法的时间窗口可能对结果至关重要。总之,这种治疗方法可能是治疗TRT可以使用的转移性肿瘤的一种有前景的方法。临床转化结果表明,与TRT结合使用时,同时而不是顺序地阻断PD-1/PD-L1轴可以提高总生存期和长期肿瘤控制。该研究主要探讨了两种放射治疗形式——外照射放疗和靶向放射性核素治疗(TRT)的区别,并提出了将TRT与免疫疗法相结合的新治疗方案。
LabEx提供的组化检测服务:
对所有五组(A-E)切除的肿瘤进行 CD31 染色以评估肿瘤血管,Ki-67 染色以评估肿瘤增殖,TUNEL 染色以确定 DNA 损伤。在有大面积坏死的 D 组和 E 组中,我们对肿瘤边缘的活细胞进行了评估。如图5、5所示,D组(同时使用177Lu-EB-RGD和抗PD-L1 mAbs治疗)的肿瘤血管范围是所有组中最小的。此外,在A、B和C组中,Ki-67 阳性染色的细胞比例相对较高,而在D组和E组中,细胞增殖明显减少。血红素和伊红染色显示(LabEx提供的服务),D组的大部分肿瘤区域在初始治疗 7 天后已经坏死,而其他四组的肿瘤仅在肿瘤中部有坏死区域,这表明坏死是由于供血不足和缺氧造成的。
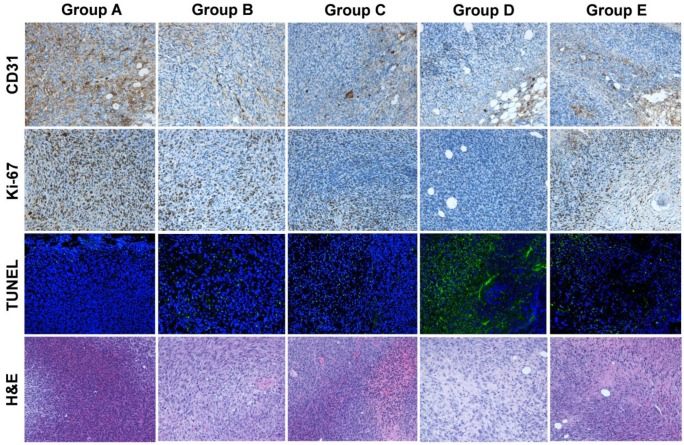
用生理盐水(A 组)治疗后,切除肿瘤的 CD31(血管)、Ki-67(增殖)、TUNEL(凋亡)以及苏木精和伊红(H&E)免疫荧光染色、 177Lu-EB-RGD(B组)、αPD-L1 mAb(C组)、177Lu-EB-RGD和αPD-L1 mAb同时联合治疗(D组)以及177Lu-EB-RGD和αPD-L1 mAb连续联合治疗(E组)。A-D组的肿瘤样本在治疗后第7天采集,E组的样本在治疗后第14天采集(αPD-L1 mAb从TRT第11天开始)。
主要发现:
- PD-1/PD-L1轴阻断在肿瘤治疗中表现出潜力,但需要与放疗等组合以提高效果。
- TRT联合PD-L1阻断可改善肿瘤治疗效果,可能通过激活肿瘤微环境的免疫反应实现。
- TRT后PD-L1表达上调,联合PD-L1阻断可增加CD8+ T细胞浸润,促进肿瘤控制。
- 同时进行TRT和PD-L1阻断比顺序治疗更有效,时间窗口关键。
- 早期的PD-L1阻断可能有助于产生持久的抗肿瘤免疫记忆。
- TRT可能通过激活肿瘤微环境的免疫反应,提高肿瘤对治疗的敏感性。
本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。







 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


